BACKGROUND: Follicular lymphoma (FL) is an indolent, incurable lymphoma with a heterogeneous clinical course characterized by multiple relapses. Recent data indicate a potential synergism between tazemetostat and lenalidomide and an additive effect with rituximab, offering a clinical rationale for this as a combination strategy (Batlevi, Blood 2022). SYMPHONY-1 Phase 1b, Stage 1 (EZH-302; NCT04224493) is an open-label, single-arm, safety run-in trial to evaluate the safety and tolerability of tazemetostat combined with lenalidomide plus rituximab (R2). This study indirectly compares the efficacy results of the SYMPHONY-1 Phase 1b single arm trial with an external control arm (ECA) derived from the Flatiron Health Research Database (FHRD), consisting of real-world FL patients treated with R2 after receiving ≥1 prior systemic therapy.
METHODS: This study utilized individual patient data from SYMPHONY-1 based on a data cutoff date of July 10, 2023, and the FHRD, a large database of electronic health records from oncology practices in the United States, including data through December 31, 2022. The treatment arm consisted of patients enrolled in the safety run-in portion of SYMPHONY-1 treated with a combination of tazemetostat plus R2, followed from enrollment to the data cutoff date. The ECA was constructed by applying eligibility criteria adapted from SYMPHONY-1 to real-world patients from the FHRD treated with R2 in second line (2L); patients were followed from the start date of the earliest eligible line of therapy (LOT) to the end of the patient's record. The primary endpoint was objective response rate (ORR), defined as the proportion of patients who achieved a complete or partial response. In SYMPHONY-1, ORR was assessed by an independent review committee according to the 2014 Lugano Classification. In the ECA, real-world ORR (rwORR) was determined by the treating clinician's interpretation of change in disease burden. The analysis set included patients from the treatment arm with ≥1 evaluable response assessment and patients from the ECA with ≥1 radiographic assessment documented in the FHRD. The statistical analysis used propensity score weighting (PSW) to adjust the distribution of seven prespecified baseline patient characteristics of the ECA to align with the treatment arm. These characteristics were age, Eastern Cooperative Oncology Group Performance Status, disease grade at diagnosis, progression of disease within 24 months (POD24) from diagnosis, lactate dehydrogenase (LDH) > upper limit of normal (ULN), size of the largest node, and Follicular Lymphoma International Prognostic Index (FLIPI) risk group. An impute-then-exclude multiple imputation strategy was used to handle missing data for variables required in the eligibility criteria and PSW. The primary endpoint was indirectly compared by estimating the difference between the ORR of the treatment arm and rwORR of the balanced ECA on each imputed dataset, and the associated standard errors were approximated using bootstrapping. Rubin's rules were applied to combine the results into a single estimate with a 95% confidence interval and corresponding two-sided p-value.
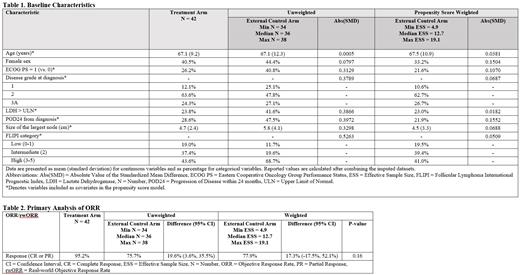
RESULTS: The primary analysis compared 42 patients in the treatment arm from SYMPHONY-1 and a median of 36 patients (range: 34 to 38) in the ECA among 815 total patients in the FHRD across ten imputed datasets. The unweighted analysis found an ORR of 95.2% in the treatment arm and 75.7% in the ECA, resulting in a difference of 19.6% (95% confidence interval [CI]: 3.6-35.5) (Table 2). After PSW, the distribution of baseline characteristics for the ECA was comparable with the treatment arm (all standardized mean differences < 0.16), and the median effective sample size was reduced to 12.7 (range: 4.9 to 19.1) (Table 1). ORR was 95.2% in the treatment arm and 77.9% in the ECA, resulting in a difference of 17.3% (95% CI: -17.5-52.1; two-sided P = 0.16) (Table 2).
CONCLUSIONS: This ECA study found that patients treated with tazemetostat combined with R2 were estimated to have a higher ORR than those treated with R2, though inference is limited due to small sample sizes. These findings help contextualize the SYMPHONY-1 Phase 1b single-arm trial results and combination potential of tazemetostat plus R2. There is a randomized Phase 3 confirmatory trial underway to confirm the clinical benefit of tazemetostat plus R2 (SYMPHONY-1 Stage 2, NCT04224493).
OffLabel Disclosure:
Nastoupil:AstraZeneca: Honoraria; Gilead Sciences/Kite Pharma: Honoraria, Research Funding; Regeneron: Honoraria; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria; AbbVie: Honoraria. Salles:Nordic Nanovector: Consultancy; Incyte: Consultancy; Kite/Gilead: Consultancy; Loxo/Lilly: Consultancy; Janssen: Consultancy, Research Funding; EPIZYME: Consultancy; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; BeiGene: Consultancy; AbbVie: Consultancy, Honoraria; Molecular Partners: Consultancy; Genmab: Consultancy; Owkin: Current holder of stock options in a privately-held company; Merck: Consultancy, Honoraria; ATB Therapeutics: Consultancy; Debiopharm: Consultancy; BMS/Celgene: Consultancy; Novartis: Consultancy; Nurix: Consultancy; Orna: Consultancy; Ipsen: Consultancy, Research Funding. Leonard:National Cancer Institute, Leukemia and Lymphoma Society, Genentech, Epizyme, Janssen: Research Funding; AbbVie, AstraZeneca, Astellas, Bayer, BeiGene, BMS, Calithera, Constellation, Eisai, Epizyme, GenMab, Grail, Incyte, Janssen, Karyopharm, Lilly, Merck, Mustang Bio, Pfizer, Roche/Genentech, Seagen, Second Genome, Sutro: Consultancy. Veazey:Ipsen Biopharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Perrot:Ipsen Pharma: Current Employment, Current equity holder in publicly-traded company. Doban:Ipsen Pharma: Current Employment, Current equity holder in publicly-traded company. Bonnet:Ipsen Pharma: Current Employment, Current equity holder in publicly-traded company. Sosinsky:Ipsen Pharma: Consultancy. Crowley:Ipsen Pharma: Consultancy. Lieb:Ipsen Pharma: Consultancy. Wang:Ipsen Pharma: Consultancy. Zeldow:Ipsen Pharma: Consultancy. Harton:Ipsen Pharma: Consultancy. Wang:Ipsen Pharma: Consultancy. Garawin:Ipsen Biopharmaceuticals: Current Employment, Current equity holder in publicly-traded company.
Tazemetostat is a methyltransferase inhibitor indicated for the treatment of: - Adult patients with relapsed or refractory follicular lymphoma whose tumors are positive for an EZH2 mutation as detected by an FDA-approved test and who have received at least 2 prior systemic therapies. - Adult patients with relapsed or refractory follicular lymphoma who have no satisfactory alternative treatment options.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal